Selective carbon–carbon bond formation: terpenylations of amines involving hydrogen transfers†
Abstract
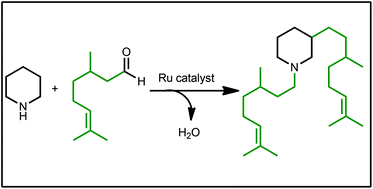
The well-defined ruthenium(II) complex A featuring a phosphine sulfonate chelate promotes the introduction of terpene moieties onto cyclic saturated amines through hydrogen “auto” transfers without side alkene reduction. These eco-friendly transformations enable the production of diverse N- and C-terpenoid alkaloids with only water and carbon dioxide as benign side products.

 Please wait while we load your content...
Please wait while we load your content...