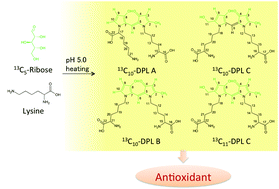
Our group has recently isolated and identified novel yellow compounds named dilysyl-dipyrrolones A (DPL A; 1) and B (DPL B; 2) in a heated aqueous solution containing xylose and lysine under weakly acidic conditions. In this study, we isolated and identified a novel DPL derivative (DPL C; 3), which has the same structure as DPL B, except for containing a hydroxymethyl group. To estimate the formation mechanism of DPL derivatives, 13C-labeled DPLs were prepared and analyzed with LC/MS and NMR. 13C-labeling experiments using [1-13C] ribose showed that the formation pathway of DPL C was different from those of DPLs A and B. In addition, 13C-labeling experiments using [U-13C5] ribose and [1-13C] lysine showed that C-6 of a methine moiety in DPL C was derived from C-5 of ribose or acetic acid in buffer. Based on these results, we postulated the formation mechanism of DPLs. We then showed that DPLs A and B had potent antioxidant activities.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...