Relationships between molecular structure and kinetic and thermodynamic controls in lipid systems. Part I: propensity for oil loss of saturated triacylglycerols
Abstract
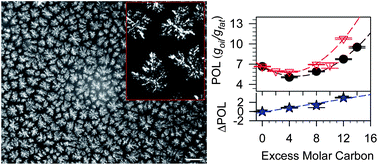
Pure saturated triacylglycerols (TAGs) in canola oil were used as model systems to analyse oil loss in structured oil both from thermodynamic and kinetic perspectives. Two important parameters which effectively and predictively measure the relative propensity of a solid network to lose/hold oil were defined: (1) the rate of oil loss, K, which is a quantified representation of the kinetics of oil loss and (2) the initial amount of oil susceptible to be lost, i.e., the propensity for oil loss (POL), which is a representation of the thermodynamics of oil binding. It was found that the POL and K values do not always trend in the same fashion, suggesting that the mechanism of oil binding is complex, depending on the structurant's crystalline form locked within the oil network. The two parameters were, however, correlated to the melting and thermal behavior of the structurants, to the polymorphic structures that are obtained during the cooling process and to the habit (shape, size and morphology) of the crystalline phase in the oil. Both POL and K had a strong correlation to the oil loss.

 Please wait while we load your content...
Please wait while we load your content...