High Seebeck coefficient redox ionic liquid electrolytes for thermal energy harvesting†
Abstract
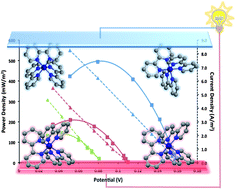
Manipulation of the cobalt(II/III) tris(bipyridyl) redox couple through anion exchange has improved its solubility in ionic liquids and 3-methoxypropionitrile (MPN). This has allowed the preparation of electrolytes with high Seebeck coefficients, Se = 1.5–2.2 mV K−1, and thereby excellent prospects for thermal harvesting. The unique physical properties of ionic liquids offer ideal characteristics for their use as electrolytes in thermoelectrochemical cells, particularly for applications involving thermal energy available at temperatures in the 100–200 °C range. The power generation characteristics of thermoelectrochemical cells using a series of ionic liquids and MPN with the CoII/III(bpy)3(NTf2)2/3 couple are described. Power densities reached >0.5 W m−2 in unoptimized devices, operating with a 130 °C hot side. The high Seebeck coefficient appears to have its origins in the high-to-low spin transition upon electron transfer in this cobalt complex.

 Please wait while we load your content...
Please wait while we load your content...