Photocatalytic water gas shift using visible or simulated solar light for the efficient, room-temperature hydrogen generation
Abstract
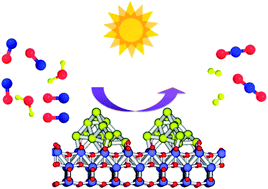
Gold nanoparticles supported on P25 TiO2 act as highly efficient photocatalysts for promoting the simultaneous H2O reduction to H2 and CO oxidation to CO2. This photocatalytic process can be performed using simulated solar light as well as light from a LED quasi monochromatic centred at 450 nm, indicating that not only UV but also visible light promotes the reaction. This novel photocatalytic reaction corresponds to the water gas shift which is an endothermic process that is currently carried out on a large industrial scale at temperatures about 350 °C. In contrast, the photocatalytic process is performed at ambient temperature with no other energy requirement than sunlight. Other related TiO2 and CeO2 photocatalysts containing noble metals (Pt or Pd) behave similarly, but with lower efficiency both in the UV and in the visible region than Au/TiO2.

 Please wait while we load your content...
Please wait while we load your content...