Synthesis, formation mechanism, and dehydrogenation properties of the long-sought Mg(NH2BH3)2 compound†
Abstract
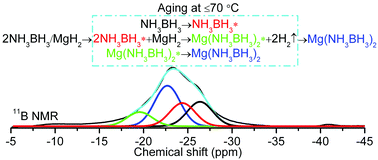
The synthesis of magnesium amidoborane, Mg(NH2BH3)2 (MgAB), has been attracting considerable interest since the recognition of the potential of metal amidoboranes as promising hydrogen storage media. But so far, all the efforts for synthesizing Mg(NH2BH3)2 using mechanochemical or wet chemistry methods have been frustrated. In this paper, we report a successful synthesis of MgAB using ammonia borane (AB) and magnesium hydride (MgH2) or magnesium (Mg) powder as starting materials. It was found that the post-milled 2AB/MgH2 and 2AB/Mg mixtures undergo solid-phase reactions under mild temperatures (≤70 °C), resulting in the formation of a new crystalline phase. A combination of X-ray diffraction (XRD), scanning electron microscope (SEM), Fourier transformation infrared (FTIR), and solid-state 11B MAS NMR characterizations, in conjunction with weight loss monitoring and the designed metathesis experiment, strongly indicates that the new crystalline phase is the long-sought MgAB. Our study using the solid-state 11B NMR technique further revealed that the formation of MgAB involves a reaction mechanism that is distinct from other metal amidoboranes. MgH2 or Mg may actually react with the mobile phase of AB (AB*), rather than with the starting normal AB. The examination of its properties revealed that MgAB is a stable compound at room temperature and can release ∼10 wt% H2 of high purity at temperature below 300 °C, suggesting that it is a promising hydrogen storage material.

 Please wait while we load your content...
Please wait while we load your content...