Corrosion of magnesium electrolytes: chlorides – the culprit
Abstract
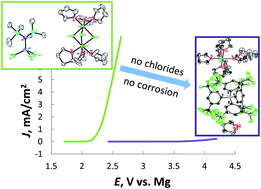
Chloride containing magnesium electrolytes are corrosive towards non noble metals. Currently the development of non-corrosive magnesium electrolytes is a key challenge on the road to a rechargeable magnesium battery. The component responsible for corrosion of magnesium electrolytes has not been previously elucidated. Here we clarify that chlorides in the

 Please wait while we load your content...
Please wait while we load your content...