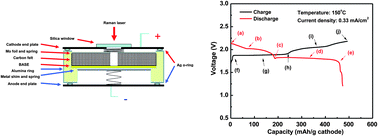
In this study, we reported an intermediate-temperature (∼150 °C) sodium–sulfur (Na–S) battery. With a relatively low operating temperature, this novel battery could reduce the cost and safety issues associated with the conventional high-temperature (300–350 °C) Na–S battery. A dense β′′-Al2O3 solid membrane and tetraglyme were utilized as the electrolyte separator and catholyte solvent in this battery. Solubility tests indicated that a cathode mixture of Na2S4 and S exhibited extremely high solubility in tetraglyme (e.g., >4.1 M for Na2S4 + 4 S). CV scans of Na2S4 in tetraglyme revealed two pairs of redox couples with peaks at around 2.22 and 1.75 V, corresponding to the redox reactions of polysulfide species. The discharge/charge profiles of the Na–S battery showed a slope region and a plateau, indicating multiple steps and cell reactions. In situ Raman measurements during battery operation suggested that polysulfide species were formed in the sequence of Na2S5 + S → Na2S5 + Na2S4 → Na2S4 + Na2S2 during discharge and in a reverse order during charge. This battery showed dramatic improvement in rate capacity and cycling stability over room-temperature Na–S batteries, which makes it more attractive for renewable energy integration and other grid related applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...