Proton-exchange mechanism of specific Cs+adsorptionvia lattice defect sites of Prussian blue filled with coordination and crystallizationwater molecules†
Abstract
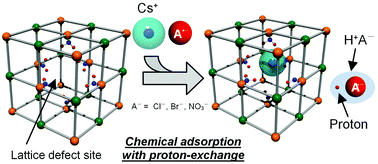
We have revealed the fundamental mechanism of specific Cs+
- This article is part of the themed collection: Coordination Programming: Science of Molecular Superstructures Towards Chemical Devices

 Please wait while we load your content...
Please wait while we load your content...