Homo- and heterobinuclear Cu2+ and Zn2+ complexes of abiotic cyclic hexaazapyridinocyclophanes as SOD mimics†
Abstract
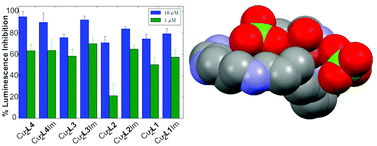
The new receptor 3,7,11,15,19,23-hexaaza-1(2,6)-pyridinacyclotetracosaphane (L1) containing a complete sequence of propylenic chains has been synthesised. The acid–base behaviour and Cu2+ and Zn2+ coordination have been analysed by potentiometric measurements in 0.15 M NaClO4 for L1 and for the related compounds 3,7,11,14,18,22-hexaaza-1(2,6)-pyridinacyclotricosaphane (L2), 3,7,10,13,16,20-hexaaza-1(2,6)-pyridinacycloheneicosaphane (L3) and 3,7,10,12,15,19-hexaaza-1(2,6)-pyridinacycloicosaphane (L4). The crystal structure of [(CuH4L2)(H2O)(ClO4)](ClO4)5·3H2O shows an interesting combination of a metal ion coordinated by the

 Please wait while we load your content...
Please wait while we load your content...