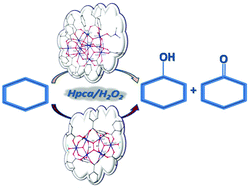
Two multinuclear complexes [Fe6(μ3-O)2(μ4-O2)L10(OAc)2(H2O)2]·2.625Et2O·2.375H2O (1) and [FeIII11Cl(μ4-O)3(μ3-O)5L16(dmf)2.5(H2O)0.5]·Et2O·1.25dmf·3.8H2O (2), where HL = 3,4,5-trimethoxybenzoic acid and dmf = dimethylformamide, have been prepared from trinuclear iron(III) carboxylates via their structural rearrangement in dimethylformamide or diethyl ether–dimethylformamide 9 : 1, respectively, and slow vapor diffusion of diethyl ether into the reaction mixture. Both compounds have been characterized by X-ray diffraction, optical, Mössbauer spectroscopy, and magnetic measurements. Complex 1 possesses a hexanuclear ferric peroxido–dioxido {Fe6(O2)(O)2}12+ core unit, which adopts a recliner conformation, while complex 2 contains an unprecedented {Fe11O8Cl}16+ core, in which 9 ferric ions are six-coordinate and the remaining two are five-coordinate. Another structural feature of note of the undecanuclear core is the presence of a deformed cubane entity {Fe4(μ3-O)(μ4-O)3}4+. Both complexes act as catalyst precursors for the oxidation of cyclohexane to cyclohexanol and cyclohexanone with aqueous H2O2, in the presence of pyrazinecarboxylic acid. Remarkable TONs and TOFs (the latter mainly for 1) with concomitant quite good yields have been achieved under mild conditions. Moreover, 1 exhibits remarkably high activity in an exceptionally short reaction time (45 min), being unprecedented for any metal catalyzed alkane oxidation by H2O2. The catalytic reactions proceed via Fenton type chemistry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...