Formation of an alkyne during degradation of metal–alkylidyne complexes†
Abstract
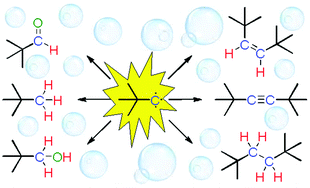
The compound [(Ot-Bu)3W![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ct-Bu] (1) (t-Bu = C(CH3)3) decomposes upon contact with water and several organic products are formed, including di-tert-butylacetylene, t-BuC
Ct-Bu] (1) (t-Bu = C(CH3)3) decomposes upon contact with water and several organic products are formed, including di-tert-butylacetylene, t-BuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ct-Bu. This process is reminiscent of the degradation of trinuclear metal–alkylidyne complexes in which free carbynes are ejected into solution, couple and form alkynes along with many other products. The reactivity pattern of the resulting t-BuC carbynes that includes extensive hydrogen abstraction, cleavage of alkynes and lack of reactivity towards alkenes is indicative of a quartet (S = 3/2) spin state. A similar spin state was assigned to other RC (R = alkyl) species that were produced by degrading M3–alkylidyne (M = transition metal) complexes in water. t-BuC
Ct-Bu. This process is reminiscent of the degradation of trinuclear metal–alkylidyne complexes in which free carbynes are ejected into solution, couple and form alkynes along with many other products. The reactivity pattern of the resulting t-BuC carbynes that includes extensive hydrogen abstraction, cleavage of alkynes and lack of reactivity towards alkenes is indicative of a quartet (S = 3/2) spin state. A similar spin state was assigned to other RC (R = alkyl) species that were produced by degrading M3–alkylidyne (M = transition metal) complexes in water. t-BuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ct-Bu is also produced during thermal decomposition of solid 1. In 1977 Fischer and co-workers reported a very similar process in which solids of Br(CO)4Cr
Ct-Bu is also produced during thermal decomposition of solid 1. In 1977 Fischer and co-workers reported a very similar process in which solids of Br(CO)4Cr![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR1 and Br(CO)4Cr
CR1 and Br(CO)4Cr![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR2 were co-thermolyzed to produce R1C
CR2 were co-thermolyzed to produce R1C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR2, R1C
CR2, R1C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR1, and R2C
CR1, and R2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR2. Fischer had considered the involvement of free carbynes in the making of the alkynes but later resorted to other explanations. The current results suggest that his original proposal is indeed valid.
CR2. Fischer had considered the involvement of free carbynes in the making of the alkynes but later resorted to other explanations. The current results suggest that his original proposal is indeed valid.

 Please wait while we load your content...
Please wait while we load your content...