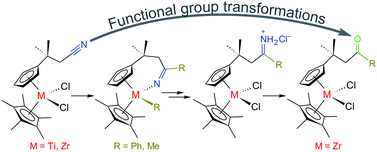
Functional group transformations at the group 4 metallocene framework have been demonstrated, which have provided relatively straightforward access to otherwise synthetically challenging derivatives. The pendant nitrile group in Ti and Zr metallocene complexes of the type [(η5-C5Me5)(η5-C5H4CMe2CH2CN)MCl2] was converted into an intramolecularly bound ketimido moiety by alkylation, which took place not only at the nitrile, but also at the metal centre. The choice of an alkylating reagent (alkyl/aryl lithium, Grignard reagent) was crucial: e.g., 2 equiv. of MeMgBr effected the alkylation only at the metal, yielding selectively complexes [(η5-C5Me5)(η5-C5H4CMe2CH2CN)MMe2], while the use of PhMgBr, PhLi, or MeLi instead gave selectively the ketimido complexes. Organyl lithium reagents were, however, not compatible with the titanocene derivatives. The metal-bound ketimides were subsequently cleaved off by the reaction with HCl, which afforded metallocene dichlorides with a pendant imino group. These compounds were easily protonated again at the nitrogen atom to produce a cationic iminium moiety. Aqueous hydrolysis of the imine or its respective hydrochloride proved to be viable in the case of Zr and it finally afforded a pendant ketone group attached to the zirconocene framework.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...