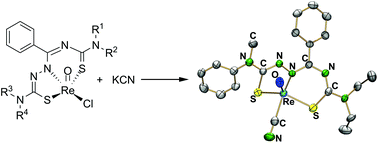
Rhenium(V) complexes containing tridentate thiosemicarbazones/thiosemicarbazides (H2L1) derived from N-[N′,N′-dialkylamino(thiocarbonyl)]benzimidoyl chlorides with 4,4-dialkylthiosemicarbazides have been synthesized by ligand-exchange reactions starting from [ReOCl(L1)]. The chlorido ligand of [ReOCl(L1)] (4) is readily replaced and reactions with ammonium thiocyanate or potassium cyanide give [ReO(NCS)(L1)] (6) and [ReO(CN)(L1)] (7), respectively. The reaction of (NBu4)[ReOCl4] with H2L1 and two equivalents of ammonium thiocyanate, however, gives in a one-pot reaction [ReO(NCS)2(HL1)] (8), in which the pro-ligand H2L1 is only singly deprotonated. An oxo-bridged, dimeric nitridorhenium(V) compound of the composition [{ReN(HL1)}2O] (11) is obtained from a reaction of (NBu4)[ReOCl4], H2L1 and sodium azide. The six-coordinate complexes [ReO(L1)(Ph2btu)] (12), where HPh2btu is N,N-diphenyl-N′-benzoylthiourea, can be obtained by treatment of [ReOCl(L1)] with HPh2btu in the presence of NEt3. Studies of the antiproliferative effects of the [ReOX(L1)] system (X = Cl−, NCS− or CN−) on breast cancer cells show that the lability of a monodentate ligand seems to play a key role in the cytotoxic activity of the metal complexes, while the substitution of this ligand by the chelating ligand Ph2btu− completely terminates the cytotoxicity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...