Efficient new constructs against triple negative breast cancer cells: synthesis and preliminary biological study of ferrocifen–SAHA hybrids and related species
Abstract
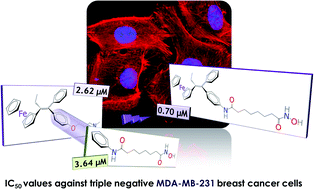
Chemotherapeutic agents combining several active groups within a single molecule can modulate multiple cellular pathways and, thus, exhibit higher efficacy than single-target drugs. In this study, six new hybrid compounds combining tamoxifen (TAM) or ferrocifen (FcTAM) structural motifs with suberoylanilide hydroxamic acid (SAHA) were synthesised and evaluated. Antiproliferative activity was first explored in cancer cell lines. Combining FcTAM and SAHA structural motifs to form the unprecedented FcTAM–SAHA hybrid molecule led to an increased cytotoxicity (IC50 = 0.7 μM) in triple-negative MDA-MB-231 breast cancer cells when compared to FcTAM or SAHA alone (IC50 = 2.6 μM and 3.6 μM, respectively), while the organic hybrid analogue TAM–SAHA was far less cytotoxic (IC50 = 8.6 μM). In hormone-dependent MCF-7 breast cancer cells, FcTAM–SAHA was more active (IC50 = 2.0 μM) than FcTAM (IC50 = 4.4 μM) and TAM–SAHA (IC50 > 10 μM), but less toxic than SAHA (IC50 = 1.0 μM). Surprisingly, FcTAM–PSA, an N1-phenylsuberamide derivative, also possessed strong antiproliferative activity (IC50 = 0.5 μM and 1.8 μM in MDA-MB-231 and MCF-7 cells, respectively). Subsequent biochemical studies indicate that estrogen receptor alpha (ERα) and histone deacetylases (HDAC) are not the main targets of the hybrid compounds for their antiproliferative effect. Interestingly, both organometallic compounds were able to induce p21waf1/cip1 gene expression in MCF-7 breast cancer cells in accordance with their antiproliferative activity.

 Please wait while we load your content...
Please wait while we load your content...