Versatile solid-state coordination chemistry of telluroether complexes of silver(i) and copper(i)†
Abstract
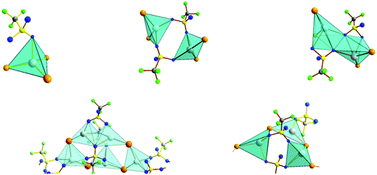
The treatment of EPh2 (E = Te, Se) with Ag(O3SCF3) or Cu(O3SCF3)·1/2C6H6 in dichloromethane yields isomorphous complexes [Ag(TePh2)3](O3SCF3) (1), [Cu(TePh2)3](O3SCF3) (2), and [Cu(SePh2)3](O3SCF3) (3). The related reaction of TeTh2 (Th = 2-thienyl, C4H3S) with Cu(O3SCF3)·1/2C6H6 affords [Cu(TeTh2)3](O3SCF3) (4). While not isomorphic with 1–3, its crystal structure bears a close relationship with them. The reaction of TeTh2 or SePh2 with Ag(O3SCF3) yields [Ag(TeTh2)2](O3SCF3) (5) and [Ag(SePh2)2](O3SCF3) (6), respectively. They form dinuclear complexes, the silver centers of which are coordinated to two terminal R2E ligands and linked together by two bridging CF3SO3− ligands. The dinuclear complexes further form supramolecular networks through π–π stacks of the aromatic rings. The treatment of Ag(O3SCF3) with Te(CH2SiMe3)2 results in the formation of a mixture containing polynuclear [Ag{Ag[Te(CH2SiMe3)2]}4]n(O3SCF3)5n (7) and [Ag{Te(CH2SiMe3)2}]n(O3SCF3)n (8). The cyclic repeating units in 7 are connected to polymeric chains by two bridging CF3SO3− ligands. 8 contains a [–Ag–Te(CH2SiMe3)2–]n polymer. There are only weak van der Waals interactions between the polymer chains of 7 and 8.

 Please wait while we load your content...
Please wait while we load your content...