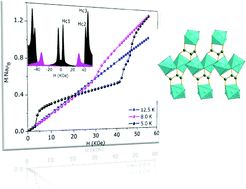
Two new layered transition metal carboxylate frameworks, [Co3(L)2(H2O)6]·2H2O (1) and [Ni3(L)2(H2O)6]·2H2O (2) (L = tartronate anion or hydroxymalonic acid), have been synthesized and characterized by X-ray single crystal analysis. Both compounds have similar 2D structures. In both compounds there are two types of metal centers where one center is doubly bridged by the alkoxy oxygen atoms through μ2-O bridging to form a 1D infinite chain parallel to the crystallographic b-axis with the corners shared between the metal polyhedra. Magnetic susceptibility measurements revealed the existence of antiferromagnetic short range correlations between Co(Ni) intra-chain metal centers (with exchange constants JCo = −22.6 and JNi = −35.4 K). At low temperatures, long range order is observed in both compounds at Néel temperatures of 11 (for 1) and 16 (for 2) K, revealing that other exchange interactions, rather than the intra-chain ones, play a role in these systems. Whereas compound 1 has an antiferromagnetic ground state, compound 2 exhibits a ferromagnetic component, probably due to spin canting. Isothermal magnetization data unveiled a rich phase diagram with three metamagnetic phase transitions below 8 K in compound 1.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...