Vanadate-dependent bromoperoxidases from Ascophyllum nodosum in the synthesis of brominated phenols and pyrroles†
Abstract
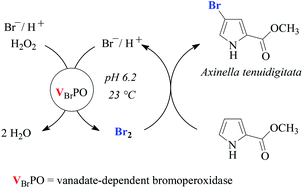
Bromoperoxidases from the brown alga Ascophyllum nodosum, abbreviated as VBrPO(AnI) and VBrPO(AnII), show 41% sequence homology and differ by a factor of two in the percentage of α-helical secondary structures. Protein monomers organize into homodimers for VBrPO(AnI) and hexamers for VBrPO(AnII). Bromoperoxidase II binds hydrogen peroxide and bromide by approximately one order of magnitude stronger than VBrPO(AnI). In oxidation catalysis, bromoperoxidases I and II turn over hydrogen peroxide and bromide similarly fast, yielding in morpholine-4-ethanesulfonic acid (MES)-buffered aqueous tert-butanol (pH 6.2) molecular bromine as reagent for electrophilic hydrocarbon bromination. Alternative compounds, such as tribromide and hypobromous acid are not sufficiently electrophilic for being directly involved in carbon–bromine bond formation. A decrease in electrophilicity from bromine via hypobromous acid to tribromide correlates in a frontier molecular orbital (FMO) analysis with larger energy gaps between the π-type HOMO of, for example, an alkene and the σ*Br,X-type LUMO of the bromination reagent. By using this approach, the reactivity of substrates and selectivity for carbon–bromine bond formation in reactions mediated by vanadate-dependent bromoperoxidases become predictable, as exemplified by the synthesis of bromopyrroles occurring naturally in marine sponges of the genera Agelas, Acanthella, and Axinella.
- This article is part of the themed collection: Vanadium in Inorganic Chemistry

 Please wait while we load your content...
Please wait while we load your content...