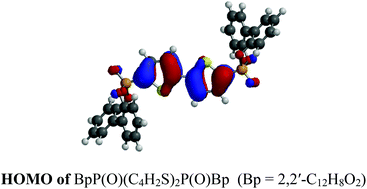
Two new series of phosphonato-substituted bithiophenes, BpP(X)(C4H2S)2H and BpP(X)(C4H2S)2P(X)Bp (Bp = 2,2′-C12H8O2, X = O, S, Se), have been synthesized and characterized using linear absorption and emission spectra, and third-order nonlinear absorption measurements at 430 nm with 27 ps laser pulses. The compounds were synthesized in three steps: (1) reacting lithiated bithiophene with (Et2N)2PCl; (2) reacting the product from the first step with biphenol; and (3) reacting the product from the second step with the appropriate chalcogen. The X-ray crystal structures of two of the compounds, BpP(O)(C4H2S)2P(O)Bp and BpP(Se)(C4H2S)2P(Se)Bp, are reported and show a number of intermolecular π–π interactions. The linear absorption spectra, emission spectra, and emission quantum yields show distinct trends with respect to the chalcogen and the number of phosphorus substituents attached to the 2,2′-bithiophene ring. The compounds show emission maxima at wavelengths ranging from 380–400 nm and, BpP(S)(C4H2S)2H shows a 23-fold increase in fluorescence quantum yield relative to that of 2,2′-bithiophene. Fluorescence lifetimes and radiative and non-radiative decay rate constants for the first singlet excited state have been extracted from the quantum yields using time-dependent DFT calculations. Nonlinear transmission measurements indicate that all of the compounds show nonlinear absorption at 430 nm with 27 ps laser pulses in spite of their low solubilities. Notably, the nonlinear absorption threshold of a 0.16 mol L−1 CH2Cl2 solution of BpP(Se)(C4H2S)2H is 0.9 J cm−2. The excellent emission quantum yields and good nonlinear absorptions make these compounds promising candidates for optical power limiting applications and as host materials for violet-blue organic light emitting diodes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...