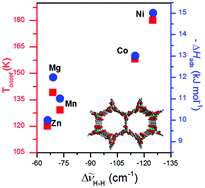
Systematic studies of H2 adsorption by variable temperature infrared (VTIR) spectroscopy have added value in the characterization of hydrogen storage materials. As a key study to describe the potential of the method, here we report VTIR spectroscopy results of H2 adsorption at isostructural MOFs CPO-27-M (M = Mg, Mn, Co, Ni, Zn). The strongest perturbation of H2 vibrational frequency is due to the interaction with an open metal site. Although ionic radius is an empirical value, the direct correlation between ionic radii of the metal cation and H2 interaction energy is found in MOFs of the same topology. The highest enthalpy of hydrogen adsorption 15 ± 1 kJ mol−1 was found for Ni2+. VTIR results of H2 adsorption at isostructural MOFs CPO-27-M (M = Mg, Mn, Co, Ni, Zn) were compared with data obtained from analogous studies performed on a large variety of microporous materials (MOFs and zeolites), underlining the relevance of the approach to get reliable energetic and entropic (ΔH0 and ΔS0) values to be compared with computational data and isosteric heats.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...