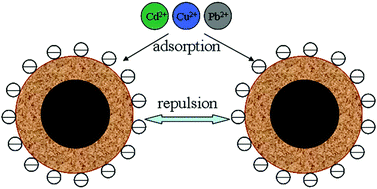
Magnetic nanomaterials that can be easily separated and recycled due to their magnetic properties have received considerable attention in the field of water treatment. However, these nanomaterials usually tend to aggregate and alter their properties. Herein, we report an economical and environmentally friendly method for the synthesis of magnetic nanoparticles with core–shell structure. MnFe2O4 nanoparticles have been successfully coated with amorphous Mn–Co oxide shells. The synthesized MnFe2O4@Mn–Co oxide nanoparticles have highly negatively charged surface in aqueous solution over a wide pH range, thus preventing their aggregation and enhancing their performance for heavy metal cation removal. The adsorption isotherms are well fitted to a Langmuir adsorption model, and the maximal adsorption capacities of Pb(II), Cu(II) and Cd(II) on MnFe2O4@Mn–Co oxide are 481.2, 386.2 and 345.5 mg g−1, respectively. All the metal ions can be completely removed from the mixed metal ion solutions in a short time. Desorption studies confirm that the adsorbent can be effectively regenerated and reused.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...