The chain-like polynuclear coordination compounds (ZnBr2)n(18-crown-6)2 (n = 4, 6, 8, 10) and [Zn5Br9][N(Tf)2] are obtained by reacting ZnBr2, SnBr4 and 18-crown-6 in the ionic liquid [(n-Bu)3MeN][N(Tf)2] (N(Tf)2: bis(trifluoromethylsulfonyl)imide). Structurally, chain-like anionic building units with corner- and edge-sharing ZnBr4/Zn(Br,O)4 tetrahedra of increasing lengths are obtained for (ZnBr2)n(18-crown-6)2. In contrast, [Zn5Br9][N(Tf)2] exhibits a cationic [Zn5Br9(18-crown-6)2]+ building unit with distorted tetrahedral, trigonal-bipyramidal and octahedral coordination of Zn2+. Besides the coordination of Zn2+ to Br−, Zn2+ is partially coordinated by 18-crown-6 with unusual folding of the crown-ether molecule. In sum, the polynuclear Zn–Br chains can be considered as intermediates between the finite [ZnBr4]2− anion and the infinite solid 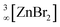 . The addition of the Lewis-acid SnBr4 turned out to be essential for product formation and results in a Br− subtraction from ZnBr2. The coordination compounds are characterized based on structure analysis, thermogravimetry and energy-dispersive X-ray analysis.
. The addition of the Lewis-acid SnBr4 turned out to be essential for product formation and results in a Br− subtraction from ZnBr2. The coordination compounds are characterized based on structure analysis, thermogravimetry and energy-dispersive X-ray analysis.
![Graphical abstract: The chain-like polynuclear coordination compounds (ZnBr2)n(18-crown-6)2 (n = 4, 6, 8, 10) and [Zn5Br9][N(Tf2)]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C3DT51130H&imageInfo.ImageIdentifier.Year=2013)
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
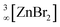 . The addition of the Lewis-acid SnBr4 turned out to be essential for product formation and results in a Br− subtraction from ZnBr2. The coordination compounds are characterized based on structure analysis,
. The addition of the Lewis-acid SnBr4 turned out to be essential for product formation and results in a Br− subtraction from ZnBr2. The coordination compounds are characterized based on structure analysis, ![Graphical abstract: The chain-like polynuclear coordination compounds (ZnBr2)n(18-crown-6)2 (n = 4, 6, 8, 10) and [Zn5Br9][N(Tf2)]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C3DT51130H&imageInfo.ImageIdentifier.Year=2013)

 Please wait while we load your content...
Please wait while we load your content...