π-Excess σ2P ligands: synthesis of biaryl-type 1,3-benzazaphosphole hybrid ligands and formation of P^P′–M(CO)4 chelate complexes†
Abstract
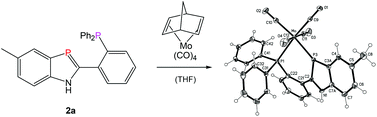
Acid-catalyzed cyclocondensations of 2-phosphanylanilines 1 with substituted benzaldehydes or heteroaryl aldehydes open a convenient route to new biaryl-type 1H-1,3-benzazaphosphole hybrid ligands 2a–f with o-phosphanylphenyl, pyridyl, imidazolyl, thienyl or o-methoxyphenyl donor groups (in addition to the σ2P donor) and to bromophenyl substituted benzazaphospholes 2g,h. Excess aldehyde leads to concomitant reductive N-alkylation, as shown by formation of 3h besides 2h. The reactions proceed via dihydrobenzazaphospholes 4, which can be detected under mild conditions. The aromaticity-driven dehydrogenation does not liberate dihydrogen but is accomplished by transfer hydrogenations, mainly by reduction of some of the aldehyde. N-Secondary 2-phosphanylanilines 5 also react with aldehydes to form the corresponding N-substituted benzazaphospholes 6. The formation of (P^P′)M(CO)4-chelate complexes 8a (M = Cr) and 9a,b (M = Mo) was demonstrated by reaction with M(CO)4(norbornadiene). The crystal structure of 9a, determined in addition to the solution structure elucidation by multinuclear NMR spectra, confirms the chelate formation and reveals a trigonal environment for the low coordinated phosphorus, with the P–Mo(0) vector bent out of the benzazaphosphole ring plane by 14.4° (0.57 Å), together with axial chirality of the molecules in the racemic crystals by twisting of the benzazaphosphole and phenyl π-planes around the common C(2)–C(21) bond.

 Please wait while we load your content...
Please wait while we load your content...