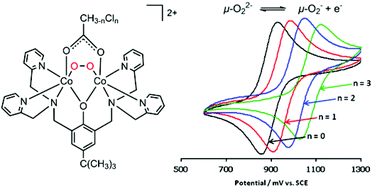
The O2 binding affinity of a series of dicobalt(II) complexes can be tuned between p(O2)50% = 2.3 × 10−3 and 700 × 10−3 atm at 40 °C by varying the number of H and Cl atoms in the bridging acetato ligands of [Co2(bpbp)(CH(3−n)ClnCO2)(CH3CN)2]2+, where bpbp− = 2,6-bis(N,N-bis(2-pyridylmethyl)aminomethyl)-4-tert-butylphenolate and n = {0, 1, 2, 3}. O2 binds most strongly to the deoxy complex containing the acetato bridge and the O2 affinity decreases linearly as the number of Cl atoms is increased from 0 to 3 in [Co2(bpbp)(O2)(CH3CO2)]2+, [Co2(bpbp)(O2)(CH2ClCO2)]2+, [Co2(bpbp)(O2)(CHCl2CO2)]2+ and [Co2(bpbp)(O2)(CCl3CO2)]2+. The O2 affinities can be qualitatively correlated with both the pKa value of the parent acetic or chloroacetic acid and the redox potential of the O22−/O2˙− couple measured for the peroxide-bridged complexes. The redox potential varies between 510 mV (vs. Fc0/+) for the acetato-bridged complex to 696 mV for the trichloroacetato-bridged system. Despite the clear difference in reactivity in solution, there are no clear trends which can be correlated to O2 affinity in the O–O bond lengths in the X-ray crystal structures at 180 K (1.415(4)–1.424(2) Å) or in the frequencies of the peroxido O–O stretch in the solid-state resonance Raman spectra at 298 K (830–836 cm−1). Using density functional theory calculations, we conclude that the Co(II) atoms of the deoxy complexes coordinate solvent molecules as auxiliary ligands and that a conformation change of the ligand is involved in the reversible O2 binding process. The alternative of five coordination in the deoxy Co(II) complexes is therefore seen as less likely. The crystal structure and p(O2)50% are also reported for the 1-naphthoato-bridged oxy complex [Co2(bpbp)(O2)(C10H7O2)]2+, and the O2 binding affinity in that case is also qualitatively consistent with the expectation from the pKa of the parent 1-naphthoic acid.
This article is Open Access
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...