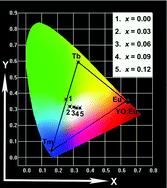
A series of Eu3+, Tb3+, Tm3+ singly and triply doped Ca3Bi(PO4)3 (CBP) phosphors were synthesized by solid-state reaction. Their structure and photoluminescence (PL) properties were first investigated in detail. Rietveld refinement analysis confirmed that the CBP has a cubic unit cell. Its space group was determined to be I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d with cell parameters a = b = c = 9.941 Å and Z = 4. In addition, the Ca3Bi(PO4)3 shows both cation disorder and oxygen sublattice disorder. For the CBP:Eu3+ phosphor, the charge transfer bands in the PLE spectra were different at 293 K and 4.3 K, and a model was presented to give a possible explanation for this phenomenon. CBP:Eu3+ shows intense red emission due to 5D0–7F2 transition and its integral emission intensity and quantum efficiency are higher than the commercial Y2O3:Eu3+ phosphor under irradiation of 397 nm, indicating that the CBP:Eu3+ phosphor might have potential application in the NUV range for solid-state lighting. The CBP:Tb3+ and CBP:Tm3+ phosphors show intense green and blue emission due to 5D4–7F2 transition of the Tb3+ ions and the 1D2–3F4 transition of the Tm3+ ions, respectively. The energy transfer from the Tb3+ to Eu3+ ions was also validated by the spectra and decay curves of the Tb3+ ions. Tunable emission colors were obtained by triply doping Eu3+, Tb3+ and Tm3+ activators in a single host and adjusting their relative ratio.
3d with cell parameters a = b = c = 9.941 Å and Z = 4. In addition, the Ca3Bi(PO4)3 shows both cation disorder and oxygen sublattice disorder. For the CBP:Eu3+ phosphor, the charge transfer bands in the PLE spectra were different at 293 K and 4.3 K, and a model was presented to give a possible explanation for this phenomenon. CBP:Eu3+ shows intense red emission due to 5D0–7F2 transition and its integral emission intensity and quantum efficiency are higher than the commercial Y2O3:Eu3+ phosphor under irradiation of 397 nm, indicating that the CBP:Eu3+ phosphor might have potential application in the NUV range for solid-state lighting. The CBP:Tb3+ and CBP:Tm3+ phosphors show intense green and blue emission due to 5D4–7F2 transition of the Tb3+ ions and the 1D2–3F4 transition of the Tm3+ ions, respectively. The energy transfer from the Tb3+ to Eu3+ ions was also validated by the spectra and decay curves of the Tb3+ ions. Tunable emission colors were obtained by triply doping Eu3+, Tb3+ and Tm3+ activators in a single host and adjusting their relative ratio.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d with cell parameters a = b = c = 9.941 Å and Z = 4. In addition, the Ca3Bi(PO4)3 shows both
3d with cell parameters a = b = c = 9.941 Å and Z = 4. In addition, the Ca3Bi(PO4)3 shows both 

 Please wait while we load your content...
Please wait while we load your content...