Self-activating nuclease and anticancer activities of copper(ii) complexes with aryl-modified 2,6-di(thiazol-2-yl)pyridine†
Abstract
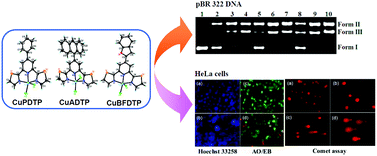
Three mononuclear copper complexes [Cu(PDTP)Cl2] (PDTP = 4-phenyl-2,6-di(thiazole-2-yl)pyridine, CuPDTP), [Cu(ADTP)Cl2] (ADTP = 4-(anthracen-9-yl)-2,6-di(thiazole-2-yl)pyridine, CuADTP) and [Cu(BFDTP)Cl2] (BFDTP = 4-(benzofuran-2-yl)-2,6-di(thiazole-2-yl)pyridine, CuBFDTP) were synthesized and characterized. The X-ray single crystallography results indicated that the Cu(II) ions showed slightly distorted square pyramid coordination environments, and the ligands deviated from ideal planarity in all three compounds. Based on the DNA binding studies, it was demonstrated that these three complexes exhibited weak DNA binding strengths, which were most likely groove binding modes. CuPDTP, CuADTP and CuBFDTP induced efficient DNA cleavage in the dark without the addition of external catalysts (oxidant or reductant). In contrast, in the presence of reducing or oxidizing agents, the nuclease activities increased more than 10-fold. Mechanistic investigations revealed the participation of reactive oxygen species, which can be trapped by ROS radical scavengers and ROS sensors. In the same experimental conditions, the free ligands and CuCl2 did not display any DNA cleaving activity. This result indicates that the complexes, rather than their components, play a significant role in the nuclease reaction process and that DNA cleavage may be initiated in an oxidative pattern. The proposed mechanism was attributed to the in situ activation of molecular oxygen by the oxidation of the copper complexes. In the MTT cytotoxicity studies, the three Cu(II) complexes exhibited an antitumor activity against the HeLa, BEL-7402 and HepG2 tumor cell lines. The HeLa cells treated with Cu(II) complexes demonstrated marked changes in their nuclear morphology, which were detected by Hoechst 33258 nuclear staining and acridine orange/ethidium bromide (AO/EB) staining assays. Nuclear chromatin cleavage also was observed from alkaline single-cell gel electrophoresis (comet assay).

 Please wait while we load your content...
Please wait while we load your content...