Chiral recognition of amino acid esters by a novel oxalic amide-linked bisporphyrin†
Abstract
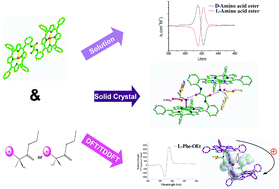
A novel oxalic amide-linked bisporphyrinate 1 has been designed and synthesized, which shows chiral recognition ability for amino acid ethyl esters. The structure of complex 1·(D-Phe-OEt)(L-Phe-OEt) has been solved by

 Please wait while we load your content...
Please wait while we load your content...