Synthesis of cubane-type Ni(ii) complexes from pyridyl-alcohol ligands; their single-molecule magnet behaviour†
Abstract
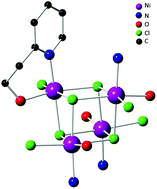
The reactions of NiCl2·2H2O or [NiCl2(DME)] (DME = dimethoxyethane) with 2-methyl-1-(pyridin-2-yl)propan-2-ol (HL) and 2-(pyridin-2-yl)ethanol (HLEt), in a 1 : 1 ratio, afforded the tetranuclear, cubane-type clusters [Ni(μ3-Cl)Cl(HL)]4 (3) and [Ni(μ3-Cl)Cl(HLEt)]4 (4), respectively. These are tetramers of the hypothetical mononuclear formula-isomers [NiCl2(HL)] and [NiCl2(HLEt)], respectively, and represent rare examples of structurally characterized cubane-like transition metal dihalogenide complexes. The 2 : 1 reactions between HL or HLEt and [NiCl2(DME)] yielded, instead, the mononuclear [NiCl2(HL)2] (1) and the dinuclear complex [Ni(μ-Cl)(HLEt)2]2Cl2 (2), respectively. The reaction of 3 with NaOH afforded selectively the cubane-type cluster [Ni(μ3-OH)Cl(HL)]4 (5) which differs only in the nature of the capping anions (Cl vs. OH, respectively). The magnetic properties of the unusual tetranuclear cubanes 3 and 5 have been investigated and revealed in both cases single-molecule magnet (SMM) behaviour.

 Please wait while we load your content...
Please wait while we load your content...