An alternative pathway for the synthesis of isocyanato- and urea-functionalised metal–organic frameworks†
Abstract
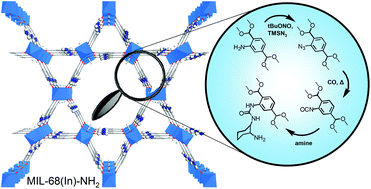
We have developed a generic two-step post-functionalisation technique for transforming amino-functionalised MOFs into their isocyanate analogues. The first part of the synthetic pathway consists in the conversion of the

 Please wait while we load your content...
Please wait while we load your content...