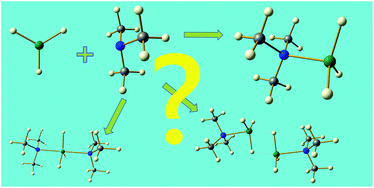
The structures adopted by a range of complexes AlH3·nL, (n = 1 or 2), have been explored in detail to identify the factors that determine the value of n, and whether a monomeric or dimeric arrangement is preferred for the 1 : 1 complexes. Single-crystal X-ray diffraction, vibrational and NMR spectroscopies, and thermal analysis data have been collected, DFT calculations have been performed for AlH3·nL species, and pKa values have been collated for a series of amine and phosphine ligands L. The pKa of the ligand L exerts an important influence on the type of complex formed: as the basicity of L increases, a monomeric structure is favoured over a dimeric arrangement. Dimeric amine complexes form if pKa < 9.76, while monomeric complexes are preferred when pKa > 9.99. The steric requirements of L also influence the structural preference: bulky ligands with large cone angles (>163°) tend to favour formation of monomers, while smaller cone angles (<125°) encourage the formation of dimeric or 1 : 2 adducts. The steric bulk of the ligand appears to be more important for phosphine complexes, with smaller phosphines being unable to stabilise the complex at ambient temperatures even through dimerisation. Raman spectroscopy and DFT calculations have been particularly helpful in elucidating the stoichiometric preferences of complexes that have been contentious; these include AlH3·NMe2Et, AlH3·NMe3 and AlH3·nEt2O.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...