The reaction of Cu(ClO4)·6H2O, Cu turnings, and 3,6-bis(2-pyridyl)-1,2,4,5-tetrazine (bptz) in Me2CO under C2H4 afforded brownish-red needle crystals of {[Cu2(bptz˙−)(C2H4)2](ClO4)}2 (1) as a minor product and brown plate crystals of [Cu2(2,5-H2bptz)(C2H4)2](ClO4)2·2Me2CO (2). The X-ray crystallographic study showed that complex 1 is composed of two independent [Cu2(bptz˙−)(C2H4)2]+ cation moieties and two ClO4− anions. In both of the cation moieties, each Cu(I) atom is coordinated by two chelate N atoms of bptz˙− and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in the trigonal-planar geometry to form a unique transoid dinuclear Cu(I)–bptz˙−/C2H4 adduct bearing a metal-stabilized tetrazine anion radical, bptz˙−, which was produced by the one-electron reduction of bptz. In contrast, complex 2 consists of one [Cu2(2,5-H2bptz)(C2H4)2]2+ cation moiety, two ClO4− anions, and two solvated Me2CO molecules. Each Cu(I) atom is coordinated by two chelate N atoms of 2,5-H2bptz and the C
C bond of C2H4 in the trigonal-planar geometry to form a unique transoid dinuclear Cu(I)–bptz˙−/C2H4 adduct bearing a metal-stabilized tetrazine anion radical, bptz˙−, which was produced by the one-electron reduction of bptz. In contrast, complex 2 consists of one [Cu2(2,5-H2bptz)(C2H4)2]2+ cation moiety, two ClO4− anions, and two solvated Me2CO molecules. Each Cu(I) atom is coordinated by two chelate N atoms of 2,5-H2bptz and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in the trigonal-planar geometry to afford a transoid dinuclear Cu(I)–C2H4 adduct with 2,5-H2bptz, which was produced by the two-electron reduction of bptz. Additionally, the similar reaction of Cu(ClO4)·6H2O, Cu turnings, and bptz in Me2CO or MeEtCO under C2H4 in the presence of ferrocene afforded orange brick crystals of [Cu2(bpdpyz)(C2H4)2](ClO4)2·Me2CO (3) and [Cu2(bpdpyz)(C2H4)2](ClO4)2 (4), respectively, together with complex 1 in higher yield. This result shows that 3,6-bis(2-pyridyl)-4,5-dihydropyridazine (bpdpyz) was produced by the cycloaddition of bptz with C2H4via a Diels–Alder addition and retro-Diels–Alder elimination of dinitrogen. In complexes 3 and 4, each Cu(I) atom is coordinated by two chelate N atoms of bpdpyz and the C
C bond of C2H4 in the trigonal-planar geometry to afford a transoid dinuclear Cu(I)–C2H4 adduct with 2,5-H2bptz, which was produced by the two-electron reduction of bptz. Additionally, the similar reaction of Cu(ClO4)·6H2O, Cu turnings, and bptz in Me2CO or MeEtCO under C2H4 in the presence of ferrocene afforded orange brick crystals of [Cu2(bpdpyz)(C2H4)2](ClO4)2·Me2CO (3) and [Cu2(bpdpyz)(C2H4)2](ClO4)2 (4), respectively, together with complex 1 in higher yield. This result shows that 3,6-bis(2-pyridyl)-4,5-dihydropyridazine (bpdpyz) was produced by the cycloaddition of bptz with C2H4via a Diels–Alder addition and retro-Diels–Alder elimination of dinitrogen. In complexes 3 and 4, each Cu(I) atom is coordinated by two chelate N atoms of bpdpyz and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 to afford an unusual cisoid dinuclear Cu(I)–C2H4 adduct. Further attempts to react excess [Cu(C2H4)n]ClO4 with bptz in Me2CO under C2H4 afforded black brick crystals of [Cu4(4,5-H2bptz)4](ClO4)4·2Me2CO (5) together with complex 1. Complex 5 is the first [4 × 4] grid-like tetranuclear Cu(I) complex constructed by the combination of Cu(I) ion and 4,5-H2bptz. The four Cu(I) atoms are spanned by four 4,5-H2bptz ligands, with two lying above the Cu4 mean plane and two lying below. In contrast, the similar reaction of excess [Cu(C2H4)n]ClO4 with bptz in Me2CO under C2H4 in the presence of ferrocene afforded reddish-brown brick crystals of {[Cu2(2,5-H2bptz)(C2H4)2](ClO4)2}2·Me2CO (6) together with complexes 1 and 5. Complex 6 is composed of two independent [Cu2(2,5-H2bptz)(C2H4)2]2+ cation moieties, four ClO4− anions, and a solvated Me2CO molecule. It is interesting that the structures of the two [Cu2(2,5-H2bptz)(C2H4)2]2+ cation moieties are different: one has a nearly planar structure and the other possesses a boat conformation owing to the bent central dihydrotetrazine ring.
C bond of C2H4 to afford an unusual cisoid dinuclear Cu(I)–C2H4 adduct. Further attempts to react excess [Cu(C2H4)n]ClO4 with bptz in Me2CO under C2H4 afforded black brick crystals of [Cu4(4,5-H2bptz)4](ClO4)4·2Me2CO (5) together with complex 1. Complex 5 is the first [4 × 4] grid-like tetranuclear Cu(I) complex constructed by the combination of Cu(I) ion and 4,5-H2bptz. The four Cu(I) atoms are spanned by four 4,5-H2bptz ligands, with two lying above the Cu4 mean plane and two lying below. In contrast, the similar reaction of excess [Cu(C2H4)n]ClO4 with bptz in Me2CO under C2H4 in the presence of ferrocene afforded reddish-brown brick crystals of {[Cu2(2,5-H2bptz)(C2H4)2](ClO4)2}2·Me2CO (6) together with complexes 1 and 5. Complex 6 is composed of two independent [Cu2(2,5-H2bptz)(C2H4)2]2+ cation moieties, four ClO4− anions, and a solvated Me2CO molecule. It is interesting that the structures of the two [Cu2(2,5-H2bptz)(C2H4)2]2+ cation moieties are different: one has a nearly planar structure and the other possesses a boat conformation owing to the bent central dihydrotetrazine ring.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in the trigonal-planar geometry to form a unique transoid dinuclear Cu(I)–bptz˙−/C2H4 adduct bearing a metal-stabilized
C bond of C2H4 in the trigonal-planar geometry to form a unique transoid dinuclear Cu(I)–bptz˙−/C2H4 adduct bearing a metal-stabilized ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 in the trigonal-planar geometry to afford a transoid dinuclear Cu(I)–C2H4 adduct with 2,5-H2bptz, which was produced by the two-electron reduction of bptz. Additionally, the similar reaction of Cu(ClO4)·6H2O, Cu turnings, and bptz in Me2CO or MeEtCO under C2H4 in the presence of
C bond of C2H4 in the trigonal-planar geometry to afford a transoid dinuclear Cu(I)–C2H4 adduct with 2,5-H2bptz, which was produced by the two-electron reduction of bptz. Additionally, the similar reaction of Cu(ClO4)·6H2O, Cu turnings, and bptz in Me2CO or MeEtCO under C2H4 in the presence of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of C2H4 to afford an unusual cisoid dinuclear Cu(I)–C2H4 adduct. Further attempts to react excess [Cu(C2H4)n]ClO4 with bptz in Me2CO under C2H4 afforded black brick crystals of [Cu4(4,5-H2bptz)4](ClO4)4·2Me2CO (5) together with complex 1. Complex 5 is the first [4 × 4] grid-like tetranuclear Cu(I) complex constructed by the combination of Cu(I) ion and 4,5-H2bptz. The four Cu(I) atoms are spanned by four 4,5-H2bptz
C bond of C2H4 to afford an unusual cisoid dinuclear Cu(I)–C2H4 adduct. Further attempts to react excess [Cu(C2H4)n]ClO4 with bptz in Me2CO under C2H4 afforded black brick crystals of [Cu4(4,5-H2bptz)4](ClO4)4·2Me2CO (5) together with complex 1. Complex 5 is the first [4 × 4] grid-like tetranuclear Cu(I) complex constructed by the combination of Cu(I) ion and 4,5-H2bptz. The four Cu(I) atoms are spanned by four 4,5-H2bptz 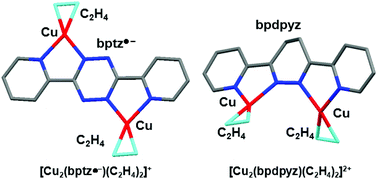

 Please wait while we load your content...
Please wait while we load your content...