Biphenyl bridged hexadentate N6-ligands – a rigid ligand backbone for Fe(ii) spin crossover complexes†
Abstract
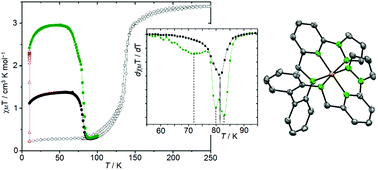
The novel hexadentate nitrogen based ligand N,N′-bis-(2-(1H-pyrazol-1-yl)pyridine-6-ylmethyl)-2,2′-biphenylenediamine (3) was synthesized and used for the preparation of iron Spin Crossover (SCO) complexes [Fe(3)][BF4]2 (4) and [Fe(3)][ClO4]2 (5), which differ only by the respective counter ion. These complex salts show different spin transition temperatures T1/2 (135 and 157 K, respectively). This effect was studied by the investigation of the solid state structure of different low- and high-spin isomers. All complexes of this series show closely related crystal packing regardless of the counter ion, metal (Zn/Fe) and spin state. The isomer exhibiting the lower transition temperature (4) was also investigated in respect to its photomagnetic behaviour. The LIESST process could be monitored for this complex, but no reverse-LIESST was observed. The relaxation of the photo-induced state occurs at ca. 80 K, showing a complex, three-state relaxation mechanism.

 Please wait while we load your content...
Please wait while we load your content...