Diverse reactivity of an isolable dialkylsilylene toward imines†
Abstract
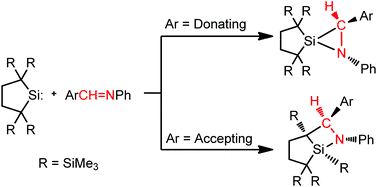
The reactions of isolable dialkylsilylene 10 with various ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh [X = H (11a), MeO (11b), and Cl (11c)] give the corresponding silaaziridines 12a–12c in high yields, which are thermally very stable and remain intact in the air and moisture for a long time. In contrast, the reactions of 10 with 4-F3CC6H4CH
NPh [X = H (11a), MeO (11b), and Cl (11c)] give the corresponding silaaziridines 12a–12c in high yields, which are thermally very stable and remain intact in the air and moisture for a long time. In contrast, the reactions of 10 with 4-F3CC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh (11d) and 3,5-(F3C)2C6H3CH
NPh (11d) and 3,5-(F3C)2C6H3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh (11e) having strong electron-withdrawing
NPh (11e) having strong electron-withdrawing

 Please wait while we load your content...
Please wait while we load your content...