Synthesis and chemistry of bis(triisopropylphosphine) nickel(i) and nickel(0) precursors†
Abstract
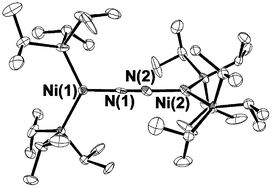
High yield syntheses of (iPr3P)2NiX (3a–c), (where X = Cl, Br, I) were established by comproportionation of (iPr3P)2NiX2 (1a–c) with (iPr3P)2Ni(η2-C2H4) (2). Reaction of 1a with either NaH or LiHBEt3 provided (iPr3P)2NiHCl (4), along with 3a as a side-product. Reduction of (iPr3P)2NiCl (3a–c) with Mg in presence of nitrogen saturated THF solutions provided the dinitrogen complex [(iPr3P)2Ni]2(μ-η1:η1-N2) (5). In aromatic solvents such as benzene and toluene a thermal equilibrium exists between 5 and the previously reported monophosphine solvent adducts (iPr3P)Ni(η6-arene) (6a,b). Reaction of 5 with carbon dioxide provided (iPr3P)2Ni(η2-CO2) (7). Thermolysis of 9 at 60 °C provided a mixture of products that included the reduction product (iPr3P)2Ni(CO)2 (8) along with iPr3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, as identified by NMR spectroscopy. Complex 8 was also prepared in high yield from the reaction of 5 with CO. Reaction of 5 with CS2 gave the dimeric carbon disulfide complex [(iPr3P)Ni(μ-η1:η2-CS2)]2 (9). Diphenylphosphine reacts with 5 to form the dinuclear Ni(I) complex [(iPr3P)Ni(μ2-PPh2)]2 (10). Complex 5 reacts with PhSH to form (iPr3P)2Ni(SPh)(H) (11), which slowly loses H2 and iPr3P to form the dimeric Ni(I) complex [(iPr3P)Ni(μ2-SPh)]2 (12) at room temperature. Complex 12 was also accessed by salt metathesis from the reaction of (iPr3P)2NiCl (3a) with PhSLi, which demonstrates the utility of 3a as a Ni(I) precursor. With the exception of 6a,b, all compounds were structurally characterized by single-crystal X-ray crystallography.
O, as identified by NMR spectroscopy. Complex 8 was also prepared in high yield from the reaction of 5 with CO. Reaction of 5 with CS2 gave the dimeric carbon disulfide complex [(iPr3P)Ni(μ-η1:η2-CS2)]2 (9). Diphenylphosphine reacts with 5 to form the dinuclear Ni(I) complex [(iPr3P)Ni(μ2-PPh2)]2 (10). Complex 5 reacts with PhSH to form (iPr3P)2Ni(SPh)(H) (11), which slowly loses H2 and iPr3P to form the dimeric Ni(I) complex [(iPr3P)Ni(μ2-SPh)]2 (12) at room temperature. Complex 12 was also accessed by salt metathesis from the reaction of (iPr3P)2NiCl (3a) with PhSLi, which demonstrates the utility of 3a as a Ni(I) precursor. With the exception of 6a,b, all compounds were structurally characterized by single-crystal X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...