Theoretical insights into covalency driven f element separations†
Abstract
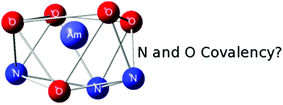
Through Density Function Theory (DFT) calculations, we set out to understand the structures and stabilities of the aqueous phase complexes [MIII(DTPA)–H2O]2− (M = Nd, Am) as well as the changes in Gibbs free energy for complexation in the gas phase and aqueous solution. All bonding analyses suggest that the preference of the DTPA5− ligand for Am over Nd is mainly due to electrostatic and covalent interactions from the oxygen atoms with the nitrogen chelates providing an additional, yet small, covalent interaction. These results question the exclusive use of hard and soft acids and bases (HSAB) concepts for the design of extracting reagents and suggest that hard-soft interactions may play more of a role in the separations process than previously thought.

 Please wait while we load your content...
Please wait while we load your content...