Impact of a dithienyl unit on photostability of N,C-chelating boron compounds†
Abstract
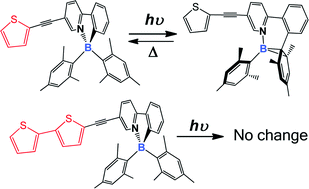
A dithienyl unit in a N,C-chelate monoboryl compound has been found to completely stabilize a N,C-chelate
- This article is part of the themed collection: Boranes and borohydrides

 Please wait while we load your content...
Please wait while we load your content...