First methoxycarbonylation of the renewable β-myrcene: high selectivity through reduced isomerisation
Abstract
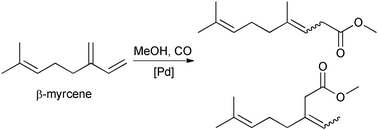
A direct route from a renewable material to odoriferous methyl esters is presented with the first methoxycarbonylation of the monoterpene β-myrcene. A simple homogeneous catalyst system of Pd(OAc)2 and the bidentate

 Please wait while we load your content...
Please wait while we load your content...