Synthesis and structures of tridentate β-diketiminato zinc phenoxides as catalysts for immortal ring-opening polymerization of l-lactide†
Abstract
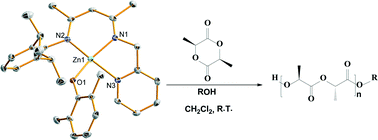
A series of new tridentate β-diketiminato zinc phenoxides were obtained by reactions of tridentate β-diketimine, different phenols and diethylzinc at room temperature, these complexes have been well characterized with 1H and 13C NMR, elemental analyses, X-ray crystallography, and electronic structure analysis by density functional theory (DFT). Experimental results show these zinc phenoxides are highly active and excellent catalysts for immortal ring-opening polymerization (ROP) of L-lactide giving isotactic poly-L-lactide with desirable molecular weights and low molecular weight distributions. Complex 2a, an electronic withdrawing zinc phenoxide complex of LZn(2,6-(CH3)2C6H3O), wherein HL = 2,6-diisopropyl-N-((2Z,4E)-4-((pyridin-2-ylmethyl)imino)pent-2-en-2-yl)aniline, can even catalyze ROP of 5000 equivalents of L-lactide under a controlled model. Experiments also revealed steric hindrance and electronic effects have significant affects in the ROP of L-lactide with these zinc phenoxides as catalysts.

 Please wait while we load your content...
Please wait while we load your content...