How metallic is gold in the direct epoxidation of propene: an FTIR study
Abstract
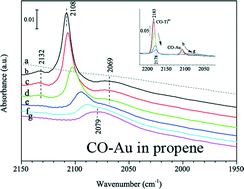
Unraveling the oxidation state of gold is important to understand the role of gold in direct propene epoxidation on gold–titania catalysts. A Fourier transform infrared study of low-temperature carbon monoxide adsorption was performed over Au/TiO2 and Au/Ti–SiO2 under an atmosphere of the reaction mixture of oxygen, hydrogen, and propene. Data reveals that the active gold sites treated by the reaction mixture are fully covered by reaction intermediates and deactivating species. Oxidation at 573 K removes these carbonaceous species on gold. Oxygen adsorption at the reaction temperature leads to positively charged gold, which can be reduced to metallic gold in the presence of hydrogen. Propene acts as an electron donor to the gold atoms resulting in negatively charged gold with the carbonyl band at 2079 cm−1. The results in this study may provide a general scheme of electron transfer via gold on the gold–titania catalysts for direct propene epoxidation.
- This article is part of the themed collection: Gold Catalysis

 Please wait while we load your content...
Please wait while we load your content...