Photocatalytic water oxidation with hematite electrodes
Abstract
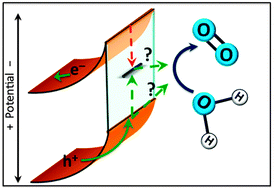
Hematite's favorable 2.1 eV band gap, valence band position, stability, abundance, and light absorption properties make it a promising semiconductor material for solar-driven water oxidation. While a mechanism for water oxidation at the surface of hematite has not yet been experimentally established, it is widely agreed upon that surface-state mediated charge recombination at the electrode–electrolyte interface competes with water oxidation. This kinetic competition ultimately limits the water splitting efficiency. The identity and role of these surface states in the water oxidation reaction is still unclear. This perspective presents recent results in probing photocatalytic water oxidation with hematite electrodes and the role of surface states. In addition, the function of surface coatings on the hematite surface, and their role as catalysts or surface passivation materials, are discussed.
- This article is part of the themed collection: Photocatalysis

 Please wait while we load your content...
Please wait while we load your content...