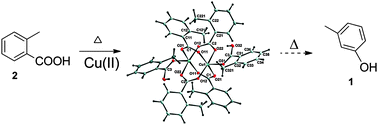
The intermediates formed during the conversion of o-toluic acid 2 to m-cresol 1 in the presence of Cu(II) according to the Keading process, together with the temperatures at which crucial changes occur, have been investigated by means of X-ray diffractometry [which confirmed the formation of the paddlewheel copper complex tetrakis(μ2-2-methylbenzoato)bis(2-methylbenzoic acid)copper(II) 5 with two apical o-toluic acid ligands], differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), NMR spectroscopy, infrared spectrometry (IR) and Maldi-Tof mass spectrometry. The major chemical change observed at 164 °C might be ascribed to the dissociation of the apical o-toluic acid ligands from 5 to give tetrakis(μ2-2-methylbenzoato)copper(II) 6 accompanied by water loss. O–C bond formation between the carboxylate oxygens and ortho-carbons of the toluic acid moieties in adjacent paddlewheels of the stepped polymeric 6 is proposed to explain the formation of copper(I) 2-methyl-6-{[(2-methylphenyl)carbonyl]oxy}benzoate 8via intermediate 7 and initiates the decomposition observed by DSC at 236 °C. Decarboxylation of 8 at 249.5 °C gives 3-methylphenyl 2-methylbenzoate 4, which can be hydrolysed to o-toluic acid 2 and the target compound, m-cresol 1.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...