Chemical approaches to study O-GlcNAcylation†
Abstract
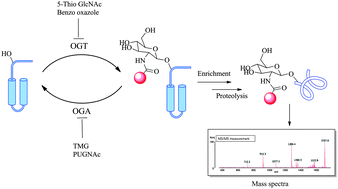
The enzymatic addition of a single β-D-N-acetylglucosamine sugar molecule on serine and/or threonine residues of protein chains is referred to as O-GlcNAcylation. This novel form of post-translational modification, first reported in 1984, is extremely abundant on nuclear and cytoplasmic proteins and has site specific cycling dynamics comparable to that of protein-phosphorylation. A nutrient and stress sensor, O-GlcNAc abnormalities underlie insulin resistance and glucose toxicity in diabetes, neurodegenerative disorders and dysregulation of tumor suppressors and oncogenic proteins in cancer. Recent advances have helped understand the biochemical mechanisms of GlcNAc addition and removal and have opened the door to developing key inhibitors towards this type of protein modification. Advanced methods in detecting and measuring O-GlcNAcylation have assisted in delineating its biological roles in a variety of cellular processes and diseased states. Availability of facile glycomic techniques are allowing for the exponential growth in the study of protein O-GlcNAcylation and are helping to elucidate key biological roles of this novel PTM.
- This article is part of the themed collection: Carbohydrate chemistry

 Please wait while we load your content...
Please wait while we load your content...