Reversible anion binding at lanthanide centres in aqueous media has emerged as an effective means of signalling and sensing the presence of selected anions. The constitution and configuration of a wide range of anion adducts has been defined by X-ray analyses and NMR methods, and both chelating and monodentate binding modes characterised. Variation of the lanthanide ion modulates charge density, and ligand modification allows alteration of both the peripheral electrostatic gradient and the local steric demand at the metal centre. Thus, selectivity for a target anion can be engineered, and the affinity constant modulated to target the desired concentration range. Changes in anion concentration can be monitored rapidly, accurately and with high spatial resolution using optical emission spectroscopy and microscopy, facilitating the measurement of anions such as bicarbonate, lactate, citrate and urate in a variety of bio-fluids.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
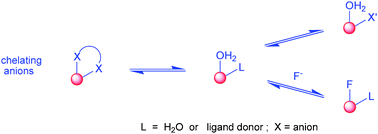

 Please wait while we load your content...
Please wait while we load your content...