The influence of transition metal oxides on the kinetics of Li2O2 oxidation in Li–O2 batteries: high activity of chromium oxides†
Abstract
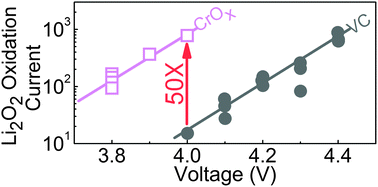
Reducing the energy loss associated with Li2O2 electrochemical oxidation is paramount to the development of efficient rechargeable lithium–oxygen (Li–O2) batteries for practical use. The influence of a series of perovskites with different eg filling on the kinetics of Li2O2 oxidation was examined using Li2O2-prefilled electrodes. While LaCrO3 is inactive for oxygen evolution upon water oxidation in alkaline solution, it was found to provide the highest specific current towards Li2O2 oxidation among all the perovskites examined. Further exploration of Cr-based catalysts showed that Cr nanoparticles (Cr NP) with an average particle size of 40 nm, having oxidized surfaces, had comparable surface area activities to LaCrO3 but much greater mass activities. Unlike Pt/C and Ru/C that promote electrolyte oxidation in addition to Li2O2 oxidation, no evidence of enhanced electrolyte oxidation was found for Cr NP relative to Vulcan carbon. X-ray absorption spectroscopy at the O K and Cr L edge revealed a redox process of Cr3+ ↔ Cr6+ on the surface of Cr NP upon Li2O2 oxidation, which might be responsible for the enhanced oxidation kinetics of Li2O2 and the reduced charging voltages of Li–O2 batteries.

 Please wait while we load your content...
Please wait while we load your content...