Molecular interactions between serum albumin proteins and Keggin type polyoxometalates studied using luminescence spectroscopy†
Abstract
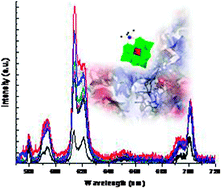
The interaction between the plenary Keggin H3PW12O40, lacunary Keggin K7PW11O39 and the Eu(III)-substituted Keggin K4EuPW11O39 (Eu-Keggin) type polyoxometalates (POMs), and the proteins human and bovine serum albumin (HSA and BSA) was studied using steady state and time-resolved Eu(III) luminescence and tryptophan (Trp) fluorescence spectroscopy. The excitation spectrum of the Eu-Keggin POM is dominated by a ligand-to-metal charge transfer band at 291 nm. In the absence of proteins, the number of water molecules coordinated in the first coordination sphere of the Eu(III) center of Eu-Keggin was determined to be 4, indicating that Eu(III) occurs as a 1 : 1 isomer in solution. In the presence of HSA or BSA, the number of coordinated water molecules decreased to 0 and 1, respectively, suggesting interaction between the Eu-Keggin POM and the protein surface. As a result of this interaction, a five-fold increase of the hypersensitive 5D0 → 7F2 transition in the luminescence intensity was observed for the Eu-Keggin–HSA complex. The association constants were calculated to be 1.5 × 102 M−1 and 2.0 × 103 M−1 for the Eu-Keggin–HSA and Eu-Keggin–BSA complexes, respectively. Tryptophan fluorescence quenching studies were performed and the quenching constants were calculated using a Stern–Volmer analysis. The obtained values of the quenching constants were 6.1 × 104 M−1 and 2.0 × 106 M−1 for the Eu-Keggin–HSA and Eu-Keggin–BSA complexes, respectively. The surface map of both proteins shows that the cavity containing the tryptophan has a positive surface potential, providing a specific binding site at the surface of albumin proteins for the negatively charged POM.

 Please wait while we load your content...
Please wait while we load your content...