Structural polymorphism in self-assembled networks of a triphenylene based macrocycle†
Abstract
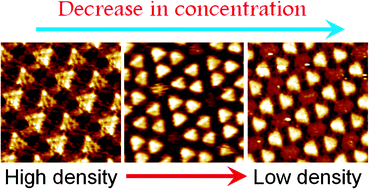
Understanding and controlling the structural polymorphism in self-assembled networks of functional molecules merit special attention. In this contribution, we describe the concentration controlled structural evolution in self-assembled monolayers of a large triangular discotic
- This article is part of the themed collection: Scanning tunneling microscopy: revealing new physical chemistry insight

 Please wait while we load your content...
Please wait while we load your content...