C72: gaudiene, a hollow and aromatic all-carbon molecule†
Abstract
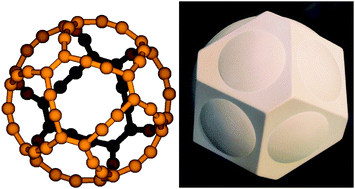
A new allotropic form of carbon is proposed. The molecular structure of a cavernous C72 molecule has been optimized at the density functional theory level. The structure belonging to the Oh point group was found to be a minimum on the potential energy surface. Current-density calculations show that the gaudiene molecule with formally 72 π electrons sustains strong diatropic currents when exposed to an external magnetic field.

 Please wait while we load your content...
Please wait while we load your content...