Plant protein interactions studied using AFM force spectroscopy: nanomechanical and adhesion properties
Abstract
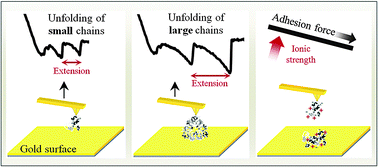
The present work was focused on the nanomechanical and adhesion properties of the napin (2S albumin) and cruciferin (12S globulin) rapeseed (Brassica napus L.)

 Please wait while we load your content...
Please wait while we load your content...