Compatibility of lithium salts with solvent of the non-aqueous electrolyte in Li–O2 batteries†
Abstract
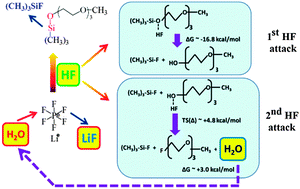
The stability of lithium salts, especially in the presence of reduced oxygen species, O2 and H2O (even in a small amount), plays an important role in the cyclability and capacity of Li–O2 cells. This combined experimental and computational study provides evidence that the stability of the electrolyte used in Li–O2 cells strongly depends on the compatibility of lithium salts with solvent. In the case of the LiPF6–1NM3 electrolyte, the decomposition of LiPF6 occurs in the cell as evidenced by in situ XRD, FT-IR and XPS analysis, which triggers the decomposition of 1NM3 solvent due to formation of HF from the decomposition of LiPF6. These reactions lead to degradation of the electrolyte and cause poor cyclability of the cell. The same reactions are not observed when LiTFSI and LiCF3SO3 are used as the lithium salts in 1NM3 solvent, or LiPF6 is used in TEGDME solvent.

 Please wait while we load your content...
Please wait while we load your content...