First principles investigation of zinc-anode dissolution in zinc–air batteries
Abstract
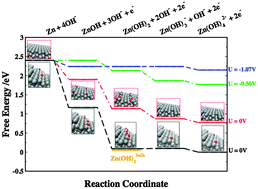
With surging interest in high energy density batteries, much attention has recently been devoted to metal–air batteries. The zinc–air battery has been known for more than a hundred years and is commercially available as a primary battery, but recharging has remained elusive, in part because the fundamental mechanisms still remain to be fully understood. Here, we present a density functional theory investigation of the zinc dissolution (oxidation) on the anode side in the zinc–air battery. Two models are envisaged, the most stable (0001) surface and a kink surface. The kink model proves to be more accurate as it brings about some important features of bulk dissolution and yields results in good agreement with experiments. From the adsorption energies of hydroxyl species and experimental values, we construct a free energy diagram and confirm that there is a small overpotential associated with the reaction. The applied methodology provides new insight into computational modelling and design of secondary metal–air batteries.

 Please wait while we load your content...
Please wait while we load your content...